Igraphite, ifomula yemolekyuli: C, ubunzima bemolekyuli: 12.01, luhlobo oluthile lwekhabhoni, i-athomu yekhabhoni nganye idityaniswe nezinye iiathom zekhabhoni ezintathu (ezicwangciswe ngamahexagons e-honeycomb) ukwenza i-molecule ye-covalent.Ngenxa yokuba iathom yekhabhoni nganye ikhupha ielectron, ezo zinokuhamba ngokukhululekileyo, ngoko ke igraphite yiconductor.
Igraphite yenye yezona zimbiwa zithambileyo, kwaye ukusetyenziswa kwayo kubandakanya ukwenza iilothe zepensile kunye nezithambiso.Ikhabhoni yinto engeyiyo eyentsimbi ebekwe kumjikelo wesibini weqela le-IVA letafile ye periodic.Igraphite yenziwa kumaqondo obushushu aphezulu.
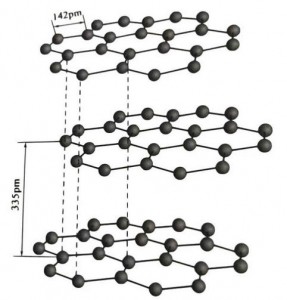
I-graphite yi-crystalline mineral element ye-carbon elements, kunye ne-crystalline lattice yi-hexagonal layered structure.Umgama phakathi komaleko womnatha ngamnye yi-3.35A, kwaye isithuba seeathom zekhabhoni kumaleko womnatha omnye yi-1.42A.Yinkqubo yekristale ene-hexagonal ene-cleavage egcweleyo.Umphezulu we-cleavage ubukhulu becala libhondi ze-molecular, ngaphantsi komtsalane kwiimolekyuli, ngoko ke ukudada kwayo kwendalo kulungile kakhulu.
Kwiikristale zegraphite, iiathom zekhabhoni zikumaleko ofanayo zenza ibhondi edibeneyo kunye ne-sp2 hybridization, kwaye iathom yekhabhoni nganye iqhagamshelwe kwezinye iiathom ezintathu kwiibhondi ezintathu ezidibeneyo.Iiathom zekhabhoni ezintandathu zenza isangqa esithandathu-eqhubekayo kwinqwelomoya enye, idlulela kwisakhiwo se-lamella, apho ubude bobhondi bebhondi yeCC yi-142pm, engaphakathi kanye kuluhlu lobude bekristale yeathom, ngoko ke kuluhlu olufanayo. , yikristale yeathom.Iiathom zekhabhoni kwinqwelomoya enye zine-p orbit enye, ezidibanayo.Ii-electron zikhululekile, zilingana nee-electron zamahhala kwiintsimbi, ngoko ke igraphite inokuqhuba ubushushu kunye nombane, olona phawu lweekristale zetsimbi.Ngaloo ndlela ikwahlelwa njengeekristale zetsimbi.
Uluhlu oluphakathi lwekristale yegraphite luhlukaniswe ngu-335pm, kwaye umgama mkhulu.Idityaniswe ne-van der Waals force, oko kukuthi, umaleko ngowekristale yemolekyuli.Nangona kunjalo, ngenxa yokuba ukudityaniswa kweeathom zekhabhoni kumaleko wenqwelomoya yomelele kakhulu kwaye kunzima kakhulu ukutshabalalisa, indawo yokuchithwa kwegraphite nayo iphezulu kakhulu kwaye iimpawu zayo zekhemikhali zizinzile.
Ngokujonga imowudi yayo ekhethekileyo yokudibanisa, ayinakuthathwa njengekristale enye okanye i-polycrystal, igraphite ngoku ithathwa njengekristale edibeneyo.
Ixesha lokuposa: Jul-31-2023